OUTLOOK THERAPEUTICS INC
Stock Chart, Company Information, and Scan Results
Stock Market Guides is not a financial advisor. Our content is strictly educational and should not be considered financial advice.
OUTLOOK THERAPEUTICS INC Company Information, Fundamentals, and Technical Indicators
Outlook Therapeutics, Inc., operates as a clinical-stage biopharmaceutical company, focuses on developing and commercializing monoclonal antibodies for ophthalmic indications. Its lead product candidate is ONS-5010, an ophthalmic bevacizumab product formulation of bevacizumab product candidate that is in Phase-III clinical trial for the treatment of wet age-related macular degeneration and other retina diseases. Outlook Therapeutics, Inc. has collaboration agreements with Cencora. The company was formerly known as Oncobiologics, Inc. and changed its name to Outlook Therapeutics, Inc. in November 2018. Outlook Therapeutics, Inc. was incorporated in 2010 and is headquartered in Iselin, New Jersey.
OUTLOOK THERAPEUTICS INC In Our Stock Scanner
As of Aug 19, 2025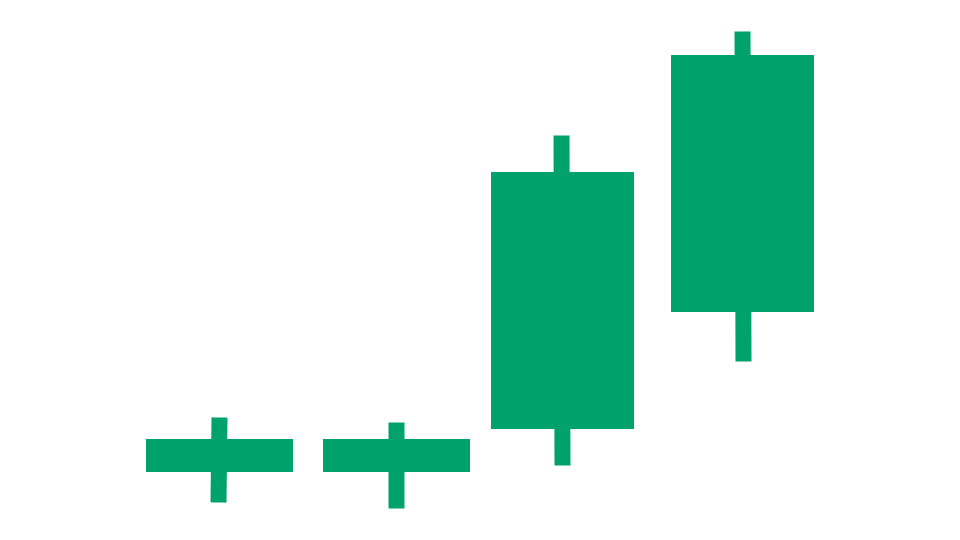




Join Our Free Email List
Get emails from us about ways to potentially make money in the stock market.